Cat #: 11-720
Zymo Research P2006 MBP-Spin Protein Miniprep Kit, Zymo Research, 10 Preps/Unit


Cat #: 11-720
Zymo Research P2006 MBP-Spin Protein Miniprep Kit, Zymo Research, 10 Preps/Unit
Zymo Research
10 Preps/Unit
Brand: Zymo Research- Fast: Purify MBP-tagged proteins from cell lysates in less than 6 minutes
- Simple: Prepare pure protein for small-scale studies using a spin-column
- Convenient: No special instrumentation needed other than a benchtop microcentrifuge
$109.90
Login To Access Your Institutional Price
Zymo Research
10 Preps/Unit
Brand: Zymo Research- Fast: Purify MBP-tagged proteins from cell lysates in less than 6 minutes
- Simple: Prepare pure protein for small-scale studies using a spin-column
- Convenient: No special instrumentation needed other than a benchtop microcentrifuge
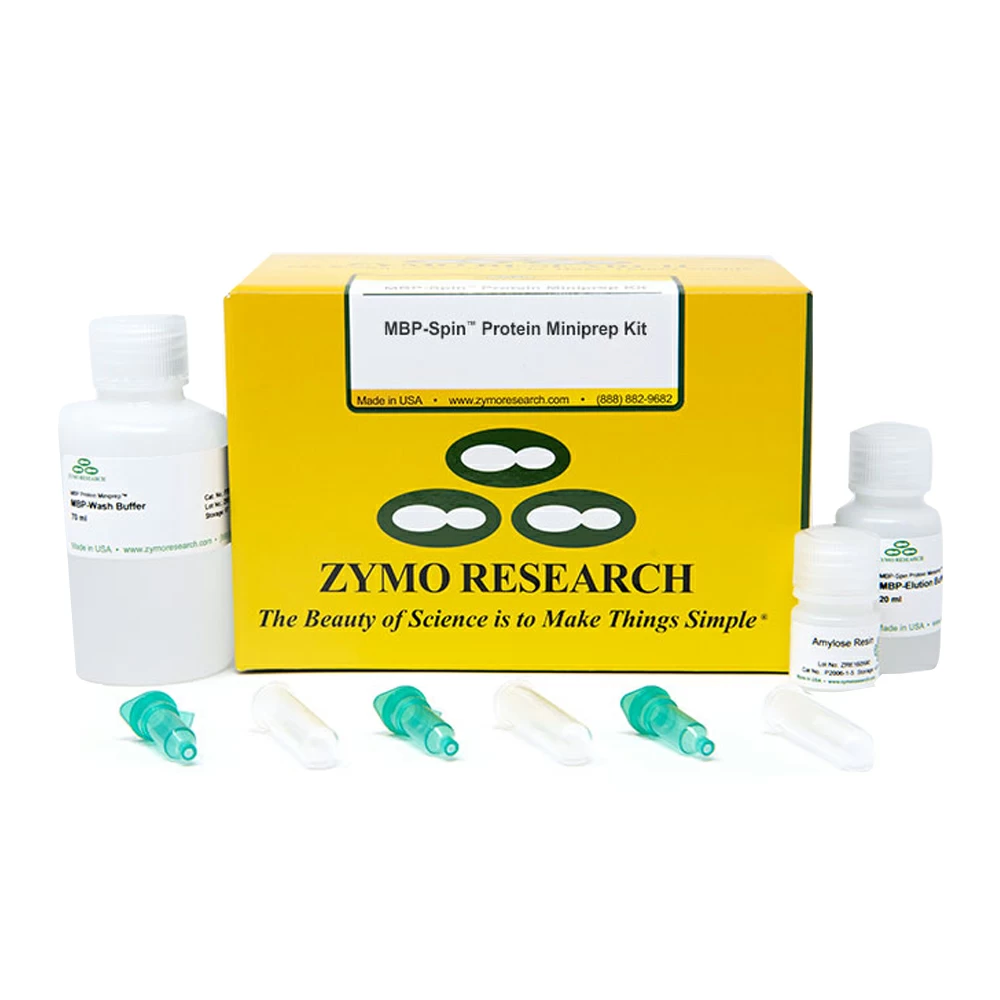
The MBP-Spin Protein Miniprep Kit provides a fast spin-column based purification technology for MBP-tagged proteins. Up to 1000µg of MBP-tagged protein can be purified in 6 minutes and eluted in MBP-Elution Buffer. The purified protein is ultra-pure and can be used directly for enzymatic assays, biochemical analyses, SDS-PAGE and other sensitive applications. The straightforward spin, wash, and elute protocol dramatically simplifies protein purification and allows the end user to get results in minutes, not hours.
| Brand | Zymo Research Corporation |
|---|---|
| Affinity Matrix | Amylose Resin |
| Binding Capacity | 100µl of Amylose Resin typically binds >/= 400µg of fusion protein. |
| Elution Method | Excess Maltose |
| Elution Volume | >/= 200µl |
| Principle of Technology | The MBP-Spin Protein Miniprep Kit uses an affinity matrix composed of amylose resin to specifically bind proteins fused to maltose-binding protein (MBP). MBP is known to significantly enhance the solubility of many proteins when fused to them. |
| Processing Time | 6 minutes |
| Product Storage | Please store the Amylose Resin at 4 °C. The other components can be stored at room temperature. |
| Purity | Electrophoretically pure and suitable for enzyme kinetics, biochemical analyses, SDS-PAGE, and other applications. |
| Required Equipment | Microcentrifuge |
| Sample Type | Cell lysates or other complex protein mixtures containing MBP-tagged proteins. |
It may still work using your own amylose solution. However, there will probably be reduced performance (less binding) compared to the supplied amylose resin.
The following concentrations of commonly used chemicals have been tested with several different proteins and did not affect protein purification: Reducing Agents 50mM beta-mercaptoethanol Non-Ionic Detergents 2% Nonidet® P-40 2% Triton® X-100 2% Tween®-20 Ionic Detergents 0.1% SDS (Sodium-N-dodecyl sulfate) Denaturing Reagents 1 M Urea (Protein Dependent) Chemicals and other Reagents 50mM EDTA 10% (v/v) Ethanol 25% (v/v) Glycerol 250mM Imidazole 1M NaCl
pH 6-9 have been evaluated successfully with several different proteins, however this may vary depending on the specific protein. Lower and higher pH have not been tested yet.
No, this will prevent the protein from binding to the matrix.
Check your buffers for signs of contamination and check the pH of the buffers. - Increase centrifugation time and speed. Ensure that the Amylose Resin drains completely after each spin (some older centrifuge models may require a longer centrifugation time). - If the problem persists, additional wash steps can be added to the purification protocol.
Optimize the lysis buffer. Reducing the detergent concentration or using a different lysis buffer might enhance yield. - Optimize the growth medium used for protein expression. Adding glucose to the media at a final concentration of 0.2% represses the expression of amylase, which interferes with protein binding to the resin. - Optimize expression conditions. Try expressing the protein at lower temperatures to increase solubility. - Reload the column. Simply repeat steps 6 and 7 until the desired volume of sample has been loaded onto the column.
The starting material contains Maltose. Free Maltose binds to the Amylose Resin and thus reduces the binding capacity for the desired protein. Too much detergent is used. Nonionic detergents or other chemicals present in the crude extract can reduce binding affinity. Some fusion proteins bind less efficiently in the present of detergents than others. Growth medium used for protein expression contains too much amylase. Cells grown in LB and similar media have substantial amounts of an amylase, which interferes with binding. Expression of insoluble protein. Overexpression of proteins may result in the formation of insoluble inclusion bodies inside cells. If a large band of over-expressed protein is visible after SDS-PAGE electrophoresis of intact cells, but not present in the cleared cell lysates, then the expressed protein may not be soluble or form inclusion bodies. Starting material is too dilute. If the starting material contains very low levels of MBP-tagged protein, then it may require more than 800µl of sample volume to purify enough protein. Simply repeat steps 6 and 7 until the desired volume has been loaded onto the column.
In the Paper from Walker et. al. 2009 (file:///Y:/Zymo Research Europe/Labor/Projekte/Tag-Spin/MBP-Spin/Literature/Walker_2010.pdf), it was shown that the MBP containing certain mutations has an enhanced binding to maltodextrin. However, in our hands we could not confirm this result. When purifying MBP-fusion proteins with or without mutations with our kit there was no difference in yield.
We do not recommend using less than 50µl of MBP-Elution Buffer. Volumes less than this will not fully saturate the resin and result in reduced elution efficiency.
Enjoy our products? Leave a review and let us know.


 Q1: Can you use your own Amylose powder resuspended in 20 % ethanol instead of the supplied Amylose resin?
Q1: Can you use your own Amylose powder resuspended in 20 % ethanol instead of the supplied Amylose resin?